8 October 2013
We read with interest the article by Natunen et al. [1] on the role of the BACE regulating factor, GGA3, in Alzheimer’s disease (AD). In this study, as has been the case with a number of other studies [2-4], the authors utilized human postmortem brain samples to show that the temporal cortex of AD patients had decreased levels of GGA3 compared to that of age-matched controls, although the decrease was not statistically significant [1]. In a separate study, Santosa et al. [3] reported a statistically significant decrease of brain GGA3 among AD patients versus healthy control subjects, but the level of brain GGA1, while decreased among AD patients, was not statistically different from control subjects. Two other studies have reported a statistically significant decrease of brain GGA1 among AD patients [4,5].
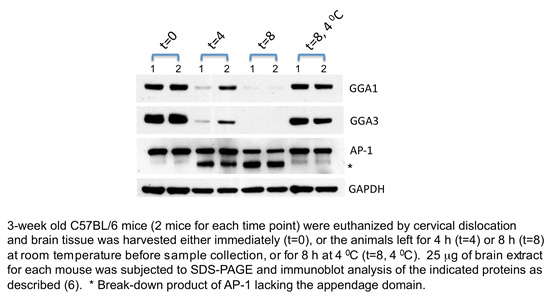
In the course of our work on the GGA proteins in mice, we uncovered an interesting property of these proteins, which we believe may be relevant to researchers in the AD field, and that might help explain some of the discrepancies between the different studies. As shown in the figure, brain levels of GGA1 and GGA3 declined substantially by 4 h after death and were almost undetectable at 8 h when the bodies were left at room temperature. The level of the clathrin adaptor, AP-1, under these same conditions was only partially reduced and GAPDH was slightly reduced. In contrast, when the bodies were kept at 4°C immediately following euthanasia, the levels of GGA1 and GGA3 remained mostly intact at the 8 h time point. Activation of caspases has been proposed as a mechanism for the intracellular degradation of human GGA1 and GGA3 [2-4] and is likely the reason why the levels of these two proteins are so dramatically reduced 8 h after death in mice since the caspase cleavage sites are conserved in these proteins between mice and humans.
Of the various studies that have looked at GGA levels in the brains of AD patients, only one report mentioned the interval between death and sample collection, which ranged from 9-72 h [3]. Moreover, it is not clear how long the deceased subjects remained at room temperature, which may have affected the results. While the levels of GGA1 and GGA3 may very well be decreased in the brains of AD patients, our data with mice indicate that caution should be exercised in the interpretation of postmortem results and that attention should be paid to the postmortem interval, should a similar process occur in humans.
Balraj Doray and Stuart Kornfeld
Department of Internal Medicine, Washington University School of Medicine, St. Louis, MO, USA
References
[1] Natunen T, Parrado AR, Helisalmi S, Pursiheimo JP, Sarajärvi T, Mäkinen P, Kurkinen KM, Mullin K, Alafuzoff I, Haapasalo A, Bertram L, Soininen H, Tanzi RE, Hiltunen M (2013) Elucidation of the BACE1 regulating factor GGA3 in Alzheimer’s disease. J Alzheimers Dis 37, 217-232.
[2] Tesco G, Koh YH, Kang EL, Cameron AN, Das S, Sena-Esteves M, Hiltunen M, Yang SH, Zhong Z, Shen Y, Simpkins JW, and Tanzi RE (2007) Depletion of GGA3 stabilizes BACE and enhances beta-secretase activity. Neuron 54, 721-737.
[3] Santosa C, Rasche S, Barakat A, Bellingham SA, Ho M, Tan J, Hill AF, Masters CL, McLean C, Evin G (2011) Decreased expression of GGA3 protein in Alzheimer's disease frontal cortex and increased co-distribution of BACE with the amyloid precursor protein. Neurobiol Dis 43, 176-183.
[4] Walker KR, Kang EL, Whalen MJ, Shen Y, Tesco G (2012) Depletion of GGA1 and GGA3 mediates postinjury elevation of BACE1. J Neurosci 32, 10423-10437.
[5] Wahle T, Thal DR, Sastre M, Rentmeister A, Bogdanovic N, Famulok M, Heneka MT, Walter J (2006) GGA1 is expressed in the human brain and affects the generation of amyloid beta-peptide. J Neurosci 26, 12838-12846.
[6] Govero J, Doray B, Bai H, Kornfeld S (2012) Analysis of Gga null mice demonstrates a non-redundant role for mammalian GGA2 during development. PLoS One 7, e30184.






