18 February 2014
Amsterdam, NL – Investigators at the University of Amsterdam, The Netherlands, have shown that progression of disease in memory clinic patients can be tracked efficiently with 45 minutes of neuropsychological testing. MRI measures of brain atrophy were shown to be less reliable to pick up changes in the same patients.
This finding has important implications for the design of clinical trials of new anti-Alzheimer drugs. If neuropsychological assessment is used as the outcome measure or “gold standard,” fewer patients would be needed to conduct such trials, or the trials may be of shorter duration.
The US Food and Drug Administration and its counterparts in other countries, such as the European Medicines Agency, require that pharmaceutical companies test and prove the effectiveness of new drugs through experimental studies. In the case of Alzheimer’s disease, this means amelioration of cognitive and behavioral symptoms or at least slowing down the rate of cognitive and behavioral decline. Until now the outcome measures in this type of research have been cognitive and behavioral rating scales, such as the Alzheimer Disease Assessment Scale (ADAS). If the effect of a new drug cannot be demonstrated with such a scale, the drug will not be approved.
The problem with scales like the ADAS is that they are quite crude and cannot pick up subtle changes, especially in early stages of the disease. As an alternative, MRI measures of brain atrophy have been proposed as outcome in clinical trials, because of allegedly better properties to detect subtle changes. This implies that fewer patients are needed in clinical trials of new drugs to show a treatment effect.
The Dutch investigators tested this claim at the memory clinic of the Academic Medical Centre, University of Amsterdam, by comparing neuropsychological assessment and MRI measures of brain atrophy in 62 patients with no or early cognitive impairment, but no dementia.
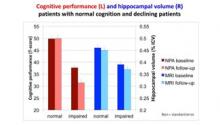
At baseline and after two years, neurologists examined the study participants and judged whether or not their cognition was normal. After two years of follow-up, twenty-eight patients were considered to be normal, and 34 had mild cognitive impairment or had progressed to dementia, mostly Alzheimer’s disease. At baseline and at follow-up all patients had a state-of-the-art MRI scan, and memory and other cognitive functions were tested with five standard neuropsychological tests.
In the group that the neurologists considered normal at follow-up, cognitive performance was indeed normal at baseline, and it remained so after two years. In the group that was considered impaired, however, cognition was already abnormal at baseline and it declined considerably over the next two years. The MRI measures concerned volumes of the left and right hippocampus, which are extremely important for memory functioning, and are the first to degenerate during the Alzheimer disease process. The volume of the hippocampus decreased less than 1% in the normal group during the follow-up interval, and more than 3% in the impaired group. The pattern of findings was similar for both techniques, but MRI showed less pronounced differences between both groups at baseline than the cognitive tests, and more importantly, less pronounced differences in rate of change.
Using figures on rates of change as collected in this study, one may calculate the numbers of patients that would be needed for a hypothetical clinical trial of a new drug. The investigators concluded that only half as many patients would be needed if neuropsychological assessment were used as the gold standard rather than MRI measures of brain atrophy. However, Dr, Edo Richard, one of the neurologists conducting the study, says, “Whichever outcome is selected, evaluation of functioning as it can be noticed by patients will always be needed to confirm the clinical relevance of any treatment effect.”
# # #
ABOUT THE JOURNAL OF ALZHEIMER’S DISEASE (JAD)
The Journal of Alzheimer's Disease is an international multidisciplinary journal to facilitate progress in understanding the etiology, pathogenesis, epidemiology, genetics, behavior, treatment and psychology of Alzheimer's disease. The journal publishes research reports, reviews, short communications, book reviews, and letters-to-the-editor. Groundbreaking research that has appeared in the journal includes novel therapeutic targets, mechanisms of disease and clinical trial outcomes. The Journal of Alzheimer's Disease has an Impact Factor of 4.17 according to Thomson Reuters' 2012 Journal Citation Reports. It is ranked #22 on the Index Copernicus Top 100 Journal List. The Journal is published by IOS Press.
ABOUT IOS PRESS
Commencing its publishing activities in 1987, IOS Press serves the information needs of scientific and medical communities worldwide. IOS Press now (co-)publishes over 100 international journals and about 130 book titles each year on subjects ranging from computer sciences and mathematics to medicine and the natural sciences.
IOS Press continues its rapid growth, embracing new technologies for the timely dissemination of information. All journals are available electronically and an e-book platform was launched in 2005.
Headquartered in Amsterdam with satellite offices in the USA, Germany, India and China, IOS Press has established several strategic co-publishing initiatives. Notable acquisitions included Delft University Press in 2005 and Millpress Science Publishers in 2008.
Contacts:
George Perry, PhD
Editor-in-Chief, Journal of Alzheimer's Disease
Dean and Professor of Biology, The University of Texas at San Antonio
Tel: +1 210 458 4450
Fax:+1 210 458 4445
E-mail: george.perry@utsa.edu
Daphne Watrin
IOS Press
Tel: +31 20 688 3355
Fax: +31 20 687 0019
E-mail: d.watrin@iospress.nl







