10 January 2022
A potential treatment for Alzheimer’s disease deserves further studies
Palo Alto, CA, USA – Current treatments for Alzheimer’s disease are of limited effectiveness and do not halt the progression of the disease and associated cognitive decline. A study, published in the Journal of Alzheimer’s Disease, showed that a novel treatment, repetitive transcranial magnetic stimulation (rTMS), may have the potential of improving the memory function in veterans with cognitive impairment due to Alzheimer’s disease.
rTMS has been previously approved by the FDA for treating patients with treatment-resistant depression. Recent studies also have shown that rTMS may improve cognition. Under the leadership of the Mental Illness Research Education and Clinical Center (MIRECC) Director and Co-Director, Drs. Jerome Yesavage and J. Kaci Fairchild, a team of researchers at VA Palo Alto conducted a pilot study to investigate the effect of rTMS on cognitive impairment in veterans with numerous medical comorbidities. Study participants underwent 20 sessions of rTMS treatment over the course of approximately 4 weeks. The study protocol is like that used for the treatment of depression. Measures of the cognitive function of these veterans (including memory, language, verbal fluency, and executive function) were acquired at baseline, end of treatment, and 4 months after the last rTMS session. The research team found that the study protocol was well-tolerated. Thirty-two veterans with cognitive impairment likely due to early Alzheimer’s disease were enrolled, 29 completed the treatment phase of the study, and 26 (13 in the active rTMS group, 13 in the sham rTMS group) completed all phases of the study and were assessed.
Analysis of study results suggested that patients in the sham group showed an expected, slight decline in memory testing over the course of the study, whereas the active treatment group showed a slight improvement at 4-months post-treatment follow up. However, the active rTMS group also demonstrated a trend in decreased verbal fluency at the end of treatment and at 4-month follow up.
Conclusion: The pilot results show that rTMS is safe in general in this cognitively impaired elderly veteran population with multiple comorbidities, and that there was a suggestion of cognitive benefit for those veterans receiving the active treatment. Given the small sample size, these effects need to be confirmed by larger studies.
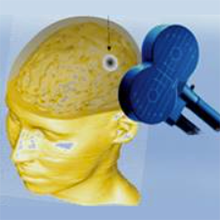
Photo caption: Schematic of a typical rTMS setup.
###
NOTES FOR EDITORS
Full study: "Repetitive Transcranial Magnetic Stimulation as a Treatment for Veterans with Cognitive Impairment and Multiple Comorbidities" by Jauhtai Cheng, J. Kaci Fairchild, M. Windy McNerney, Art Noda, J. Wesson Ashford, Trisha Suppes, Steven Z. Chao, Joy Taylor, Allyson C. Rosen, Timothy C. Durazzo, Laura C. Lazzeroni, and Jerome Yesavage (DOI: 10.3233/JAD-210349), published online in the Journal of Alzheimer’s Disease in advance of the publication of Volume 85, Issue 4. The article is available at: content.iospress.com/articles/journal-of-alzheimers-disease/jad210349.
The study is registered with clinicaltrials.gov, identified as NCT02621424 (study PI: Dr. Jauhtai Cheng).
Contact
Further information is available from the media contact Michael Hill-Jackson, VA Palo Alto Healthcare System (michael.hill-jackson@va.gov, +1 6504447380).
About the Journal of Alzheimer’s Disease
Now in its 24th year of publication, the Journal of Alzheimer’s Disease (JAD) is an international multidisciplinary journal to facilitate progress in understanding the etiology, pathogenesis, epidemiology, genetics, behavior, treatment, and psychology of Alzheimer’s disease. The journal publishes research reports, reviews, short communications, book reviews, and letters-to-the-editor. Groundbreaking research that has appeared in the journal includes novel therapeutic targets, mechanisms of disease, and clinical trial outcomes. JAD has a Journal Impact Factor of 4.472 according to Journal Citation Reports (Clarivate, 2021). The journal is published by IOS Press. j-alz.com
About IOS Press
IOS Press is an independent international scientific, technical, medical (STM) publishing house established in 1987 in Amsterdam. We produce around 90 journals and 70 books annually in a broad range of subject categories, primarily specializing in biomedical and life sciences (including neurosciences, medical informatics, cancer research, rehabilitation) and physical sciences (including computer sciences, artificial intelligence, engineering). In addition, we offer specialized services that support scientific advancement. iospress.com







