16 September 2020
We have well received the research published by Youn et al. that reports the potentiality of plasma amyloid-β (Aβ) oligomerization levels as a diagnostic biomarker in Alzheimer’s disease (AD) [1]. Many studies have claimed that there is a positive correlation between the risk of AD and plasma oligomeric Aβ (OAβ) levels [2-4]. Generally, the prevalence of AD increases with age, which leads to the hypothesis that the occurrence of cases with higher levels of plasma oligomerization tendency level increases with age. Therefore, this study aimed to measure the plasma Aβ oligomerization tendency level from samples of normal individuals of various age groups with no current AD symptoms in order to study the association between age and Aβ oligomerization tendency level.
A total of 145 asymptomatic, community-based normal healthy subjects aged 20 and older were enrolled in this study. The number of individuals in the group aged 20-29 (20s, mean age: 27.7±2.1), the group aged 30-39 (30s, mean age: 36.3±2.8), the group aged 40-41 (40s, mean age: 46.0±3.1), and the group aged 50 and older (50≤, mean age: 59.5±7.0) were 40, 40, 30 and 35, respectively. There were 74 men and 71 women.
A blood test based on the proprietary Multimer Detection System (inBlood™OAβ test, PeopleBio, Inc., South Korea), a modified Enzyme-Linked Immunosorbent Assay (ELISA) using capturing antibodies and epitope-overlapping detection antibodies for the selective detection of OAβ over Aβ monomers was used to measure the Aβ oligomerization tendency levels in the plasma. Data were analyzed using GraphPad Prism 5.01 (GraphPad, La Jolla, CA, USA). Differences between groups were analyzed using Kruskal-Wallis test. Wilcoxon signed rank tests were used for comparison between the two groups. The study was conducted in accordance with the guidelines of the Declaration of Helsinki, and was approved by the Ethics Committee of Seegene Medical Foundation, Seoul, Korea (SMF-IRB-2020-011).
The plasma Aβ oligomerization tendency levels in the 20s, 30s, 40s, and 50≤ groups were 0.43±0.09 ng/mL, 0.45±0.11 ng/mL, 0.58±0.15 ng/mL, and 0.60±0.12 ng/mL, respectively (Table 1, Fig. 1). There was no difference between the 20s and 30s, but the differences were statistically significant between the 20s and 40s, 20s and 50≤, 30s and 40s, and 30s and 50≤, respectively (p < 0.001). Plasma Aβ oligomerization tendency levels were increased with age from 40s. There was no difference between males and females, but women over the age of 40 were higher but not statistically significant (data not shown). The total average level was 0.51±0.14 ng/mL and the reference interval was 0.77 ng/mL for the right-side 95% of the normal range.
Table 1. The plasma amyloid-β oligomerization tendency levels of four different age groups.
| Age | N | Mean ± SD (ng/mL) | p |
| 20~29c,d | 40 | 0.43 ± 0.09 | <0.0001** |
| 30~39c,d | 40 | 0.45 ± 0.11 | |
| 40~49a,b | 30 | 0.58 ± 0.15 | |
| 50 ≤a,b | 35 | 0.60 ± 0.12 |
**Kruskal-Wallis test
aSignificantly different with 20~29 by Wilcoxon signed rank test (p < 0.05)
bSignificantly different with 30~39 by Wilcoxon signed rank test (p < 0.05)
cSignificantly different with 40~49 by Wilcoxon signed rank test (p < 0.05)
dSignificantly different with 50 ≤ by Wilcoxon signed rank test (p < 0.05)
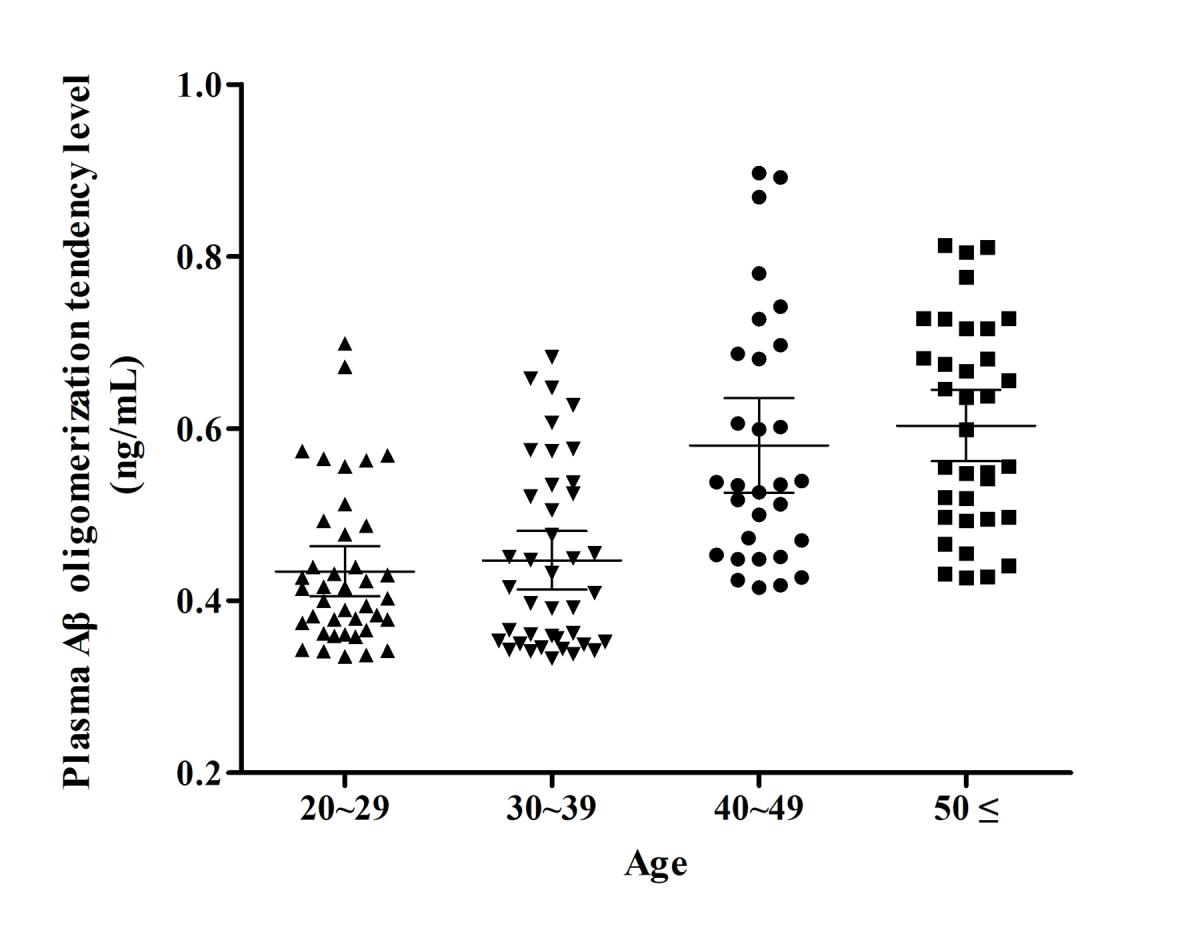
Fig. 1. The plasma amyloid-β (Aβ) oligomerization tendency levels based on the age group. The means of the plasma amyloid-β oligomerization tendency levels were increased with age in groups. Bars indicate mean with 95% confidence interval. Kruskal-Wallis test (p < 0.0001).
The analysis showed that plasma Aβ oligomerization tendency levels increase with age. In particular, the groups aged 40 and older showed statistical increases. This is presumed to be brought on by the increase in OAβ production or decrease in OAβ clearance due to the spike in chronic conditions and the drop in metabolism in individuals over the age of 40 [5]. It is likely for the resulting surplus of OAβ to travel through the blood-brain barrier and accumulate in the brain. A recent review provided evidence that AD pathological changes are triggered by amyloidopathy caused by peripheral Aβ that travels through the CNS [6]. Therefore, the findings in this study imply that there is an association between the cause of increasing AD prevalence rate with age and the increase in plasma Aβ oligomerization tendency levels.
Youn et al. set the cut-off for the plasma Aβ oligomerization tendency levels at 0.78 ng/mL, which is consistent with the normal range of this study. In addition, other studies show that the plasma Aβ oligomerization tendency levels increase across subject levels of community-based normal healthy subjects, subjective cognitive decline, mild cognitive impairment, and AD [3]. Based on this, it would be a viable option to assess AD risk by monitoring Aβ oligomerization tendency levels in normal individuals.
In conclusion, this study showed that plasma Aβ oligomerization tendency levels tend to increase with age. This points to the fact that individuals in higher age ranges are likely to have higher plasma Aβ oligomerization tendency levels, which corresponds with the increased occurrence of AD with respect to age. Therefore, measuring plasma Aβ oligomerization tendency levels would be helpful in assessing AD risk.
Dohsik Minn, Soohyun Kim
Department of Diagnostic Immunology, Seegene Medical Foundation, Seoul, Korea. E-mail: dsmin@mf.seegene.com
REFERENCES
[1] Youn YC, Lee BS, Kim GJ, Ryu JS, Lim K, Lee R, Suh J, Park YH, Pyun JM, Ryu N, Kang MJ, Kim HR, Kang S, An SSA, Kim S (2020) Blood amyloid-beta oligomerization as a biomarker of Alzheimer's disease: a blinded validation study. J Alzheimers Dis 75, 493-499.
[2] Wang MJ, Yi S, Han JY, Park SY, Jang JW, Chun IK, Kim SE, Lee BS, Kim GJ, Yu JS, Lim K, Kang SM, Park YH, Youn YC, An SSA, Kim S (2017) Oligomeric forms of amyloid-beta protein in plasma as a potential blood-based biomarker for Alzheimer's disease. Alzheimers Res Ther 9, 98.
[3] Youn YC, Kang S, Suh J, Park YH, Kang MJ, Pyun JM, Choi SH, Jeong JH, Park KW, Lee HW, An SSA, Dominguez JC, Kim S (2019) Blood amyloid-beta oligomerization associated with neurodegeneration of Alzheimer's disease. Alzheimers Res Ther 11, 40.
[4] Zhou L, Chan KH, Chu LW, Kwan JS, Song YQ, Chen LH, Ho PW, Cheng OY, Ho JW, Lam KS (2012) Plasma amyloid-beta oligomers level is a biomarker for Alzheimer's disease diagnosis. Biochem Biophys Res Commun 423, 697-702.
[5] Wang J, Gu BJ, Masters CL, Wang YJ (2017) A systemic view of Alzheimer disease - insights from amyloid-beta metabolism beyond the brain. Nat Rev Neurol 13, 612-623.
[6] Pyun JM, Kang MJ, Ryoo N, Suh J, Youn YC, Park YH, Kim S (2020) Amyloid metabolism and amyloid-targeting blood-based biomarkers of Alzheimer's disease. J Alzheimers Dis 75, 685-696.
Comments
Response Letter
Blood-based biomarkers will be the holy grail in the field of Alzheimer’s disease (AD) research and practice, once the adequacy of their sensitivity and specificity is proven. In addition, an ideal diagnostic biomarker for AD should detect the fundamental features of the molecular pathogenesis of AD. Therefore, reliable blood-based biomarkers of AD should be derived from amyloid-β (Aβ).
According to recent studies, peripheral Aβ-related markers are suitable for the evaluation of cerebral amyloidopathy even at the preclinical stage of AD. There are three types of blood-based, amyloid-targeting AD markers. The first method involves measuring Aβ related molecules (Aβ1-42, Aβ1-40, or other APP fragments) and calculate their ratios. Highly sensitive ELISA techniques or specific mass spectrometry are being used for this. The second is measuring the ratio of α-form to β-form amyloid in the peripheral circulation. The last technique is observing increasing tendencies of oligomeric forms of Aβ in plasma after spiking synthetic Aβ peptides.
The comment written by Dr. Minn & Dr. Kim noted that this oligomerization tendency is shows a slight increase with age in healthy normal subjects in his study. This is a very important finding we should keep in mind. As glycated hemoglobin (HbA1C) for diabetes mellitus, this oligomerization tendency of Aβ in plasma measured by MDS-OAβ could be used as a monitoring marker for preclinical AD pharmacotherapy in the near future. In this situation, Dr. Minn & Dr. Kim’s findings are very important to establish the management guideline according to age.
There are two possible explanations for the increased oligomerization tendencies by age; one is the possibility that there were some preclinical AD patients in the older healthy normal subject group. Because cerebral amyloidopathy may begin 15~20 years before the onset of clinical symptoms, and amyloid PET studies also found some positive cases without clinical symptoms at older population group.
Another possibility is that there may be physiological increases of Aβ oligomerization tendencies according to age. We may explain this increasing tendency by increased production of Aβ or many factors related to oligomerization, or decreased clearance of Aβ or decreased inhibiting factors of oligomerization. However, at the moment, there isn’t enough evidence to definitively explain the mechanism; more time is needed for additional research regarding this.
In conclusion, this comment including good data showing the increased oligomerization tendency of Aβ in plasma by age is a good reference upon which to establish diagnosis or treatment guidelines using MDS-OAβ.
SangYun Kim1, Young Chul Youn2
1 Department of Neurology, Seoul National University College of Medicine, Seoul, Korea
Clinical Neuroscience Center of Seoul National University Bundang Hospital, Seongnam-si, Korea
2Department of Neurology, Chung-Ang University College of Medicine, Seoul, Republic of Korea






- Comment
|